Have you ever felt short of breath? Your chest tightens and your respiratory rates increases sharply, Despite all your attempts to increase the inhaled air, it is not enough to support your needs. As exorbitant this narrative might sound, polluted air increases the difficulty to breathe properly. Yet we do not give air pollution the proper importance and coverage it requires. People tend to think that as it cannot be seen, it is not important enough. If only we were aware of what we are constantly putting inside our lungs, concern towards it would increase.

Air pollution is responsible for the deaths of thousands of people every day around the world. It is slightly reassuring that governments are increasing the pressure on outdoor pollutants levels. Contrary, the presence of pollutants in indoor air is commonly ignored by regulators. On top of that, indoor air is frequently more polluted than outdoor air. The presence of pollutants indoors is generally 2 to 5 times higher than outdoors.
Humans spend 90% of their time indoor. This together with the poor air quality indoor raises the concern to develop strategies to improve indoor air conditions. Indoor Air Quality exactly pursues this goal. Practitioners aim to continuously analyze and monitor the presence of pollutants indoor to improve conditions to healthy standards.
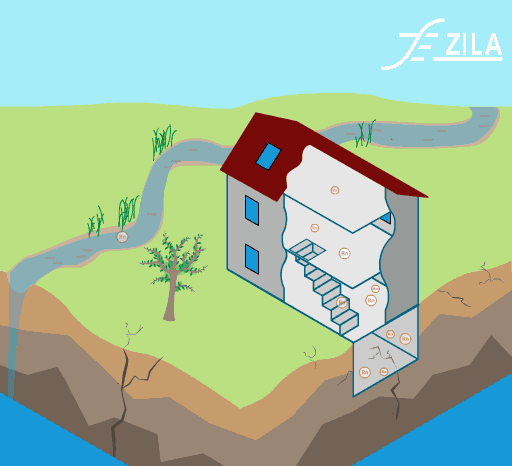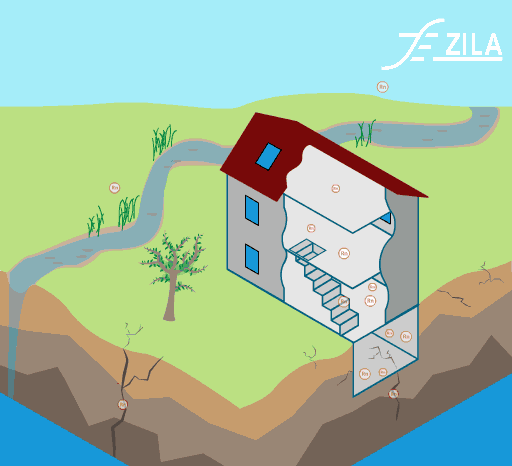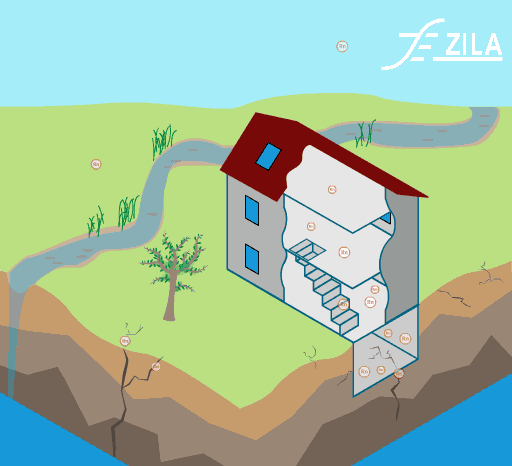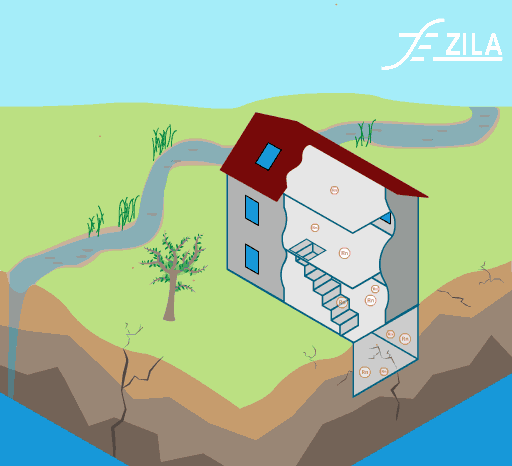
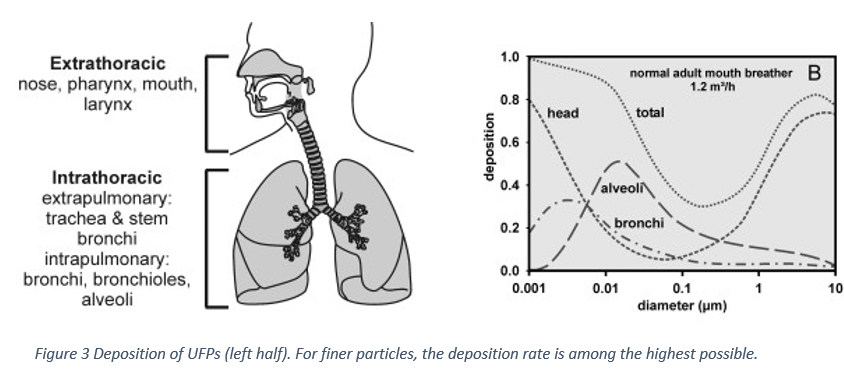
Invisible to the human eye, we are exposed to a large range of indoor pollutants. Gaseous pollutants and particulate matter can be easily found in conventional homes. Their effects on human heath can vary from light headaches and irritation of the respiratory airways to more severe affection like cancer.
One of the main drivers of Indoor Air Quality is Air Humidity. Why? You may ask. Air humidity has a direct effect on the proliferation of airborne pollutants. In this sense, they will develop faster and potentially increase the harm induced to the occupants of buildings, if the Air Humidity conditions are inappropriate. In addition, Air Humidity can play a decisive role in comfort perception.
The effects both on our health and comfort raises the alarm about the importance of Air Humidity control to improve living conditions.
Comfort perception
Humans’ wellbeing and performance is influenced by all the environmental conditions that surround us. If we want to work up to high standards, among other factors, especial attention should be given to the thermal comfort conditions. This criterion determines the temperature, relative humidity and air movement that ensures occupants are satisfied with the thermal environment.
The perception of comfort may vary from one person to another. Therefore, finding the conditions that ensures that all the rooms occupants will feel comfortable is a tedious and impossible task. The strategy should focus on providing comfortable conditions to the largest number of occupants.
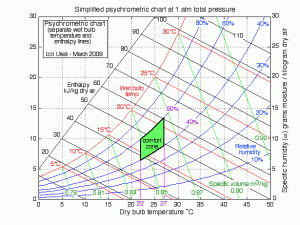
The psychometric chart, a graphical representation of the thermal conditions of the air, comes as extremely useful to represent the comfort zone. The conditions that ensure that the least number of complaints regarding the air estate are received. In this line, HVAC systems must ensure that the Relative Humidity should be between 40% and 60% and the temperature between 22ºC and 27ºC. Ensuring that the air conditions of a room is within these margins is primordial for a good air quality perception. Moving away from this area, i.e., reducing or increasing the Relative Humidity, will lead to the reduction of comfort.
The effects of low Relative Humidity (levels lower than 30% RH) can be rapidly detectable. In such conditions, tear production is reduced and consequently, eyes will feel dryer than usual. Similar effects will be experienced in the respiratory airways. Moreover, the mucus found in the upper airways will be dehydrated, reducing the efficacy of a natural protection barrier against infections. The effects of low Air Humidity and vocal perturbations have also been covered by researched. It has been concluded that air dryness may lead to an increase in the perceived effort needed for vocal production.
High levels of RH can have a strong effect on workers’ performance. Sweat is the mechanism that our body uses to reduce body temperature. Once sweat evaporates, it reduces the temperature. If high relative humidity levels (levels higher than 70% RH) are found, sweat will not be able to evaporate. Consequently, workers will feel hotter and will be more prone to suffer from exhaustion and dehydration.
Relative humidity also has an influence on the perception of Indoor Air Quality. Researchers have identified that, given the same level of pollutant concentrations, there is a correlation between perceiving lower IAQ conditions and greater Relative Humidity conditions. This effect was diffused as pollutant concentrations sharply increase.
Human health effects
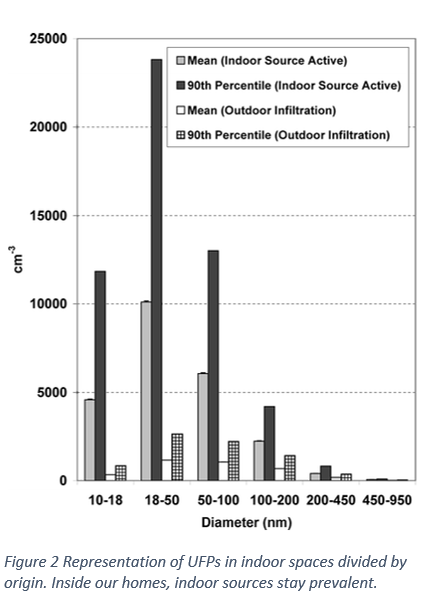
The rate at which chemical reactions occur and the survival of airborne pathogens are dependent on the Indoor Air Humidity Level. Identifying and enhancing the most adequate Relative Humidity level is not trivial due to the large number of species found indoor. Figure 2 perfectly illustrates the main affections that can be identified in indoor spaces and their effect relative to Relative Humidity. The Relative humidity that leads to an increase in the effects of these pathogens and chemical reactions vary from one to another. There is only one range, Relative Humidity between 40% and 60%, that ensure a balance between all the conditions identified. Ensuring Relative Humidity within this range does not mean the air will be free from pathogens. It will only ensure their survival at normal temperature is reduced.
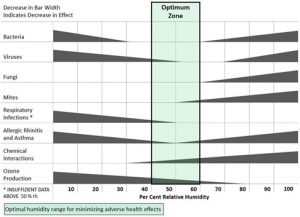
Covid-19 has played a major influence in our society over the last 2/3 years and still does today. The pandemic radically changed the way we lived, leading to an increase in the amount of time spent indoor. All our activities suddenly became indoor. Leisure activities were confined to small apartments, and working from home promptly became the norm. Therefore, special attention was given to find the measures to increase Air Quality and reduce the prevalence of the virus indoors. Previous studies had demonstrated how an increase in Relative Humidity would positively impact viruses decay rates. Based on these studies, research was performed, and the correlation was proven specifically for COVID-19 (Figure 3). Consequently, there was a growing movement demanding the World Health Organization to regulate Relative Humidity levels between 40% and 60%.

Is indoor Air Quality regulated?
Regulation in Europe and America seem to go in the same line. In installations that do not include dehumidification and/or humidification capabilities, lower and upper Relative Humidity levels are not limited. Standard 62.1, American design guidelines, only requires practitioners to ensure a Relative Humidity lower than 65% in installations that include a mechanical ventilation systems. European Standards (EN 16798-1) highlight that there is no need to include humidification and dehumidification systems. In case they are present in a room, Relative Humidity Levels should be kept between 20% and 30%.
Conclusion
It has been proved that there is a correlation between Indoor Air Quality and Air Humidity. The effects on our perception of the air quality within a room is an important matter to address. Improper Relative Humidity can lead to discomfort and the increase of the propagation of other airborne pathogens.
A range of Relative Humidity that ensures occupants healthy and comfort (40%-60%) was determined. However, the main standards (American and European) do not seem to give the needed importance to maintaining proper Relative Humidity levels. At the same time, the proposed guidelines are not precise enough and leave room for personal interpretation. Reconsideration of the Standards should be carried out to accommodate them to human needs and healthy.
References
The 40-60 rule: Why relative humidity is necessary for our health. (2021, March 29). Retrieved May 1, 2022, from https://www.condair.com/humidifiernews/blog-overview/the-40-60-rule-why-relative-humidity-is-necessary-for-our-health
Arundel, A. V., Sterling, E. M., Biggin, J. H., & Sterling, T. D. (1986). Indirect health effects of relative humidity in indoor environments. Environmental Health Perspectives, 65, 351. https://doi.org/10.2307/3430203
Fang, L., Clausen, G., & Fanger, P. O. (1998). Impact of temperature and humidity on the perception of indoor air quality. Indoor Air, 8(2), 80–90. https://doi.org/10.1111/j.1600-0668.1998.t01-2-00003.x
Kelly-Linden, S. N. J. (2020, November 2). Who urged to set global guidelines on indoor humidity to curb covid. The Telegraph. Retrieved April 29, 2022, from https://www.telegraph.co.uk/global-health/climate-and-people/urged-set-global-guidelines-indoor-humidity-curb-covid/
Koep, T. H., Enders, F. T., Pierret, C., Ekker, S. C., Krageschmidt, D., Neff, K. L., Lipsitch, M., Shaman, J., & Huskins, W. C. (2013). Predictors of indoor absolute humidity and estimated effects on influenza virus survival in grade schools. BMC Infectious Diseases, 13(1). https://doi.org/10.1186/1471-2334-13-71
Michael Rosone • May 7, 2020. (2020, October 7). Is too much humidity hurting your health? Arista. Retrieved April 29, 2022, from https://aristair.com/blog/is-too-much-humidity-hurting-your-health/
Rehva journal 02/2021 – effects of indoor air humidity. REHVA. (n.d.). Retrieved May 1, 2022, from https://www.rehva.eu/rehva-journal/chapter/effects-of-indoor-air-humidity
U.S. Environmental Protection Agency. (n.d.). Indoor Air Quality. EPA. Retrieved April 28, 2022, from https://www.epa.gov/report-environment/indoor-air-quality#pollutants
Wolkoff, P. (2018). Indoor air humidity, air quality, and health – an overview. International Journal of Hygiene and Environmental Health, 221(3), 376–390. https://doi.org/10.1016/j.ijheh.2018.01.015



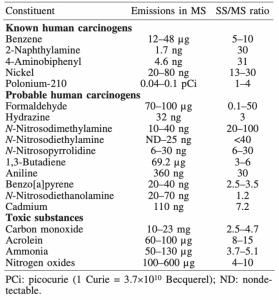
 Image 1: Common toxins found in ETS
Image 1: Common toxins found in ETS